Materials Engineering and Characterization
We conduct research in the area of Materials Engineering though close collaborations with original component manufacturers and materials technology companies. Together we characterize and evaluate the entire material design chain. From how the processes lead to structure, to how properties relate to performance.
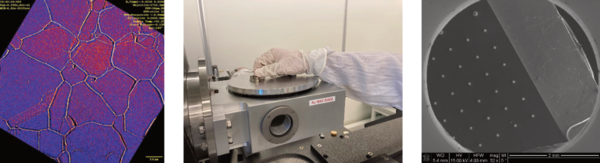
Advanced Characterization
In our facilities we have several visible light microscopes, including a Alicona InfiniteFocus 3D topology optical microscopy. Perfect for measuring geometry, surface finish, and tolerances. Around campus we have access to several Scanning and Transmission Electron Microscopy (SEM and TEM) with X-ray Energy Dispersion Spectroscopy (XEDS) and diffraction (EBSD and SAED). Samples are typically prepared as polished cross-sections or by Focused Ion Beam (FIB) milling. We work closely with partner research groups who specialize in Synchrotron and Neutron techniques, often utilizing the local world leading facilities MAX IV and ESS. Together we develop methods and perform in-operando experiment that show changes in chemistry, microstructure, and strain on industrial material in dynamic settings.
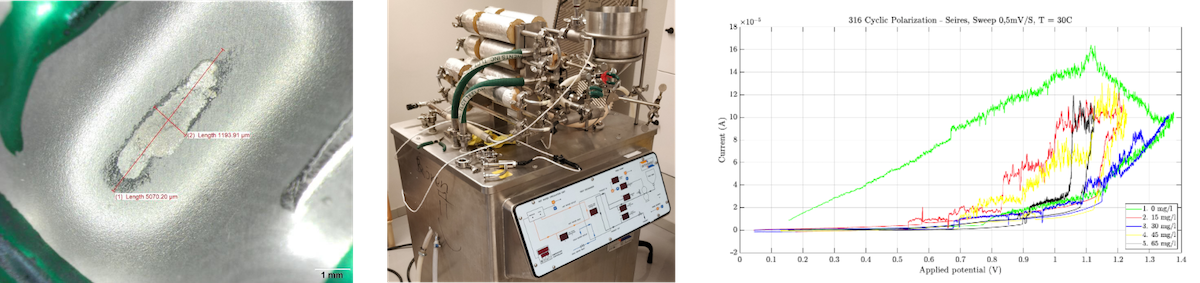
Corrosion
Our research on corrosion spans almost the entire TRL chain, with particular focus on the low and high ends. On the high end, we have refurbished industrial processing equipment, for example an ArmField pasteurization machine, to equip them for monitoring corrosion progression or to include accelerated electrochemical corrosion. Here we can study different materials in different industrial environments. On the low TRL end, we use facilities such as MAX IV to study corrosion mechanisms in new ways. By using extremely surface sensitive techniques, the data from the very thin passive oxides can be isolated and its role identified.
Joining and Bonding
The two main focus areas for our research into joining and bonding are understanding mechanism in existing joining techniques, and developing new technical concepts. We collaborate with companies who manufacture welded, brazed and diffusion bonded products. By advanced characterization and detailed analysis of commercial products and company R&D materials, we lay a chemical and microstructural puzzle to map the sequence of events that take place during joining. Our new concept revolve around using chemical gases to induce liquid metal phases in the joining zones. Our research into this new technology spring from, and greatly benefit from, the world leading research into nano material fabrication here in Lund.
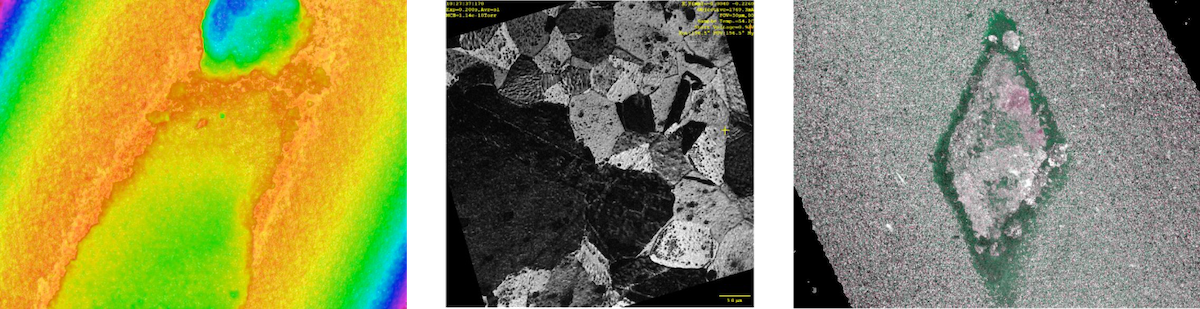
Property-Interaction-Performance
We study the tool-workpiece interactions to find wear mechanism and formation of Tool Protections Layers (TPLs). We do not limit ourselves to the commonly described abrasive or adhesive wear, but also consider chemical and diffusive wear. Here, chemical reactions occur in the contact zones which in some scenarios lead to the formation of new mechanically weak phases that trigger abrasive wear. In other scenarios a diffusional loss of vital elements, for example Co in cemented carbide or N and B in cBN, occurs which leads to a decomposition of the tool. The formation of TPLs is a version of chemical wear, however, here the newly formed phase is benefitable, either as a mechanically stronger phase or a diffusional barrier that hinders excessive diffusional loss. We also study chemical and physical interactions in joining, such as welding and brazing, and material in corrosive environments.

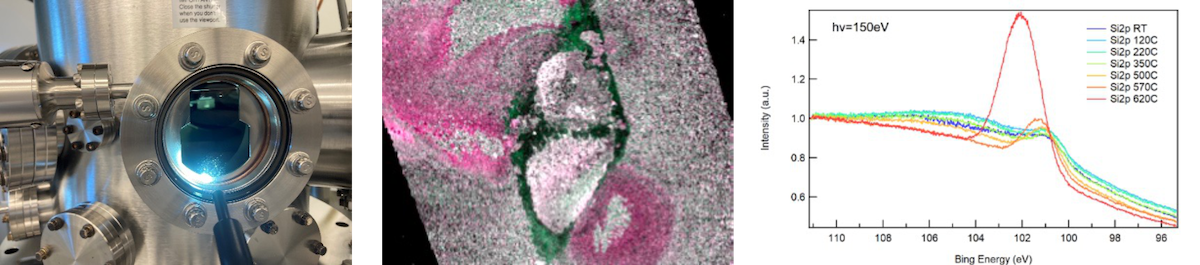
Typical Results
Results from this research include observations of differences in chemical behavior along the rake and flank of the tool in operations, deoxidation mechanisms on industrial alloys in brazing applications, strain mapping of braze joints in tension, and pitting corrosion mechanisms. Examples from machining research include a study where we showed that carbides only formed on the rake-chip interface but not on the flank-workpiece interface. In another study we showed intermetallic Co-, Ti- and W- phases formed in different regions along the contact zone. As an example on deoxidation, we have demonstrated that the amorphous Cr2O3 layer on stainless steel-316 starts to break down at around 620°C in ultra-high vacuum. This happened by a gradual transition from Cr2O3 to metallic Cr, while elemental Si oxidizes instead. Evaporation of SiO2 then followed, leaving only metallic Cr, Ni and Fe on the surface at 850°C.
Filip Lenrick, filip.lenrick@iprod.lth.se, 2022
